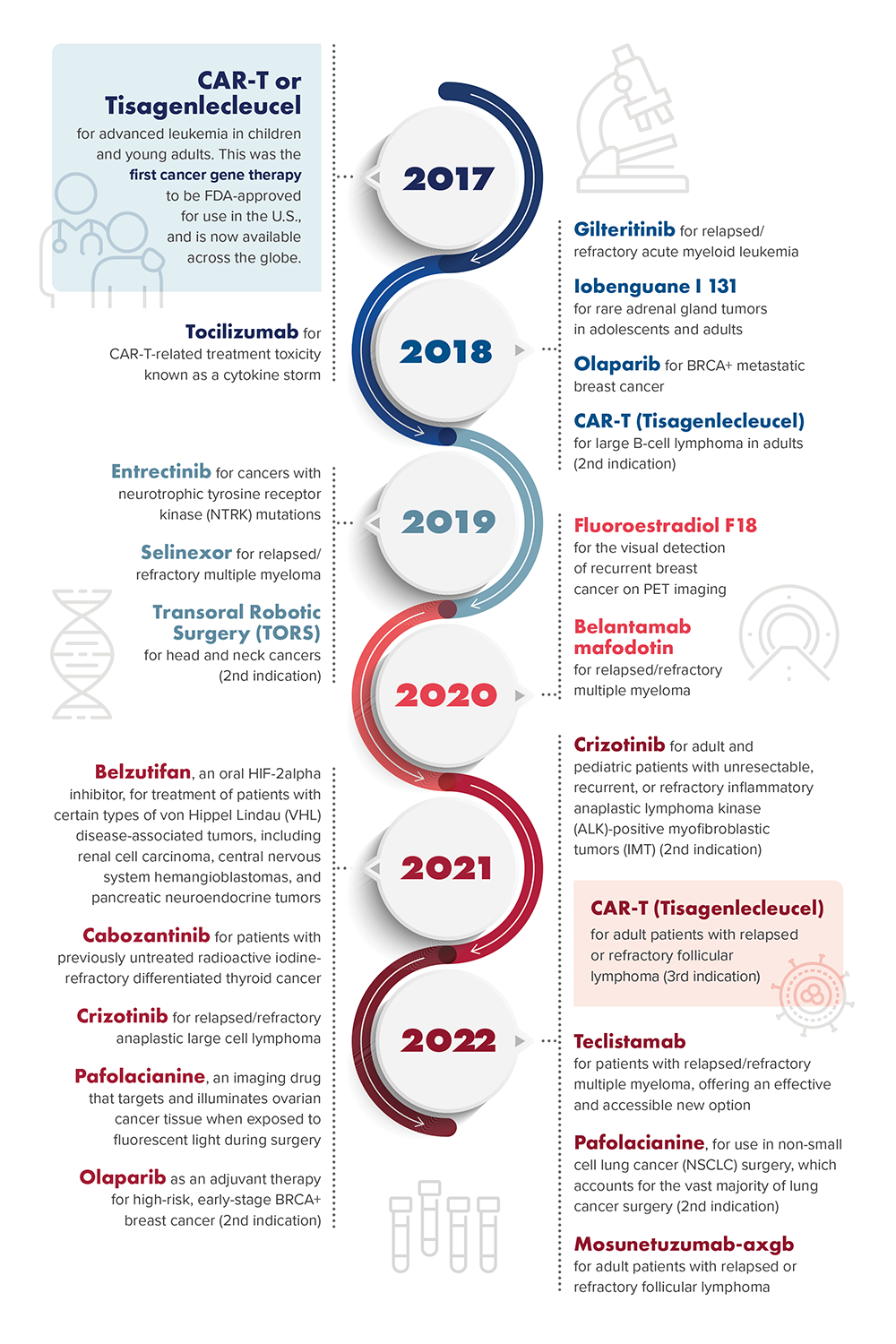
Fda Approvals Calendar
Fda Approvals Calendar - See drugs@fda for information about all of cder’s approved drugs and biological products. Therapy for hereditary angioedema (hae). Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Updated daily, it includes pdufa dates for 2025, fda advisory meetings,. Below is the list of important regulatory dates for all. Up to date information on the latest fda drug approvals. Cder drug and biologic approvals for calendar year 2023; The fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in decades, to a. Almost half of all novel medications approved by the u.s. Novel drugs are new drugs never before approved or marketed in the u.s. 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. Pdufa dates (fda approval) for all us publicly listed biotech. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. “the fda has now approved three biosimilar insulin products to treat diabetes,” said peter stein, m.d., director of the office of new drugs in the fda’s center for drug evaluation. Cder drug and biologic approvals for calendar year 2023; Below is the list of important regulatory dates for all. The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Updated daily, it includes pdufa dates for 2025, fda advisory meetings,. “the fda has now approved three biosimilar insulin products to treat diabetes,” said peter stein, m.d., director of the office of new drugs in the fda’s center for drug evaluation. Novel drugs are new drugs never before approved or marketed in the u.s. Access our free pdufa calendar to. Almost half of all novel medications approved by the u.s. The fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in decades, to a. Pdufa target dates are dates by which the fda aims to deliver their. See drugs@fda for information about all of cder’s. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. Includes list of most recent approvals, the conditions approved for, and the approval history. Novel drugs are new drugs never before approved or. The fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in decades, to a. Updated daily, it includes pdufa dates for 2025, fda advisory meetings,. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Below. Pdufa dates (fda approval) for all us publicly listed biotech. Pdufa target dates are dates by which the fda aims to deliver their. “the fda has now approved three biosimilar insulin products to treat diabetes,” said peter stein, m.d., director of the office of new drugs in the fda’s center for drug evaluation. We are in the process of updating. Food and drug administration (fda) are orphan drugs. The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. Includes list of most. See drugs@fda for information about all of cder’s approved drugs and biological products. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. 1, 2024, the fda began implementing a reorganization impacting many. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Almost half of all novel medications approved by the u.s. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Therapy for hereditary angioedema (hae).. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. We are in the process of updating fda.gov content to reflect these changes. 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. Pdufa. Below is the list of important regulatory dates for all. 1, 2024, the fda began implementing a reorganization impacting many parts of the agency. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. See drugs@fda for information about all of cder’s approved drugs and biological products. Pdufa dates (fda approval) for all us. Food and drug administration (fda) are orphan drugs. Updated daily, it includes pdufa dates for 2025, fda advisory meetings,. Cder drug and biologic approvals for calendar year 2022; We are in the process of updating fda.gov content to reflect these changes. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. A list of the anticipated fda approvals for pharmaceuticals and medical devices in the month of january 2025. Below is the list of important regulatory dates for all. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. Cder drug and biologic approvals for. The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). Pdufa target dates are dates by which the fda aims to deliver their. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. “the fda has now approved three biosimilar insulin products to treat diabetes,” said peter stein, m.d., director of the office of new drugs in the fda’s center for drug evaluation. Cder drug and biologic approvals for calendar year 2023; Updated daily, it offers investors insights into stock.FDA Calendar FDA Tracker
FDA Calendar FDA Tracker
FDA Drug Approval Process Timeline
Fda Approvals 2025 Calendars Dannie Teodora
Fda Drug Approval Calendar
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Fda Approval Calendar 2023 Everything You Need To Know August 2023
Fda Approval Calendar Qualads
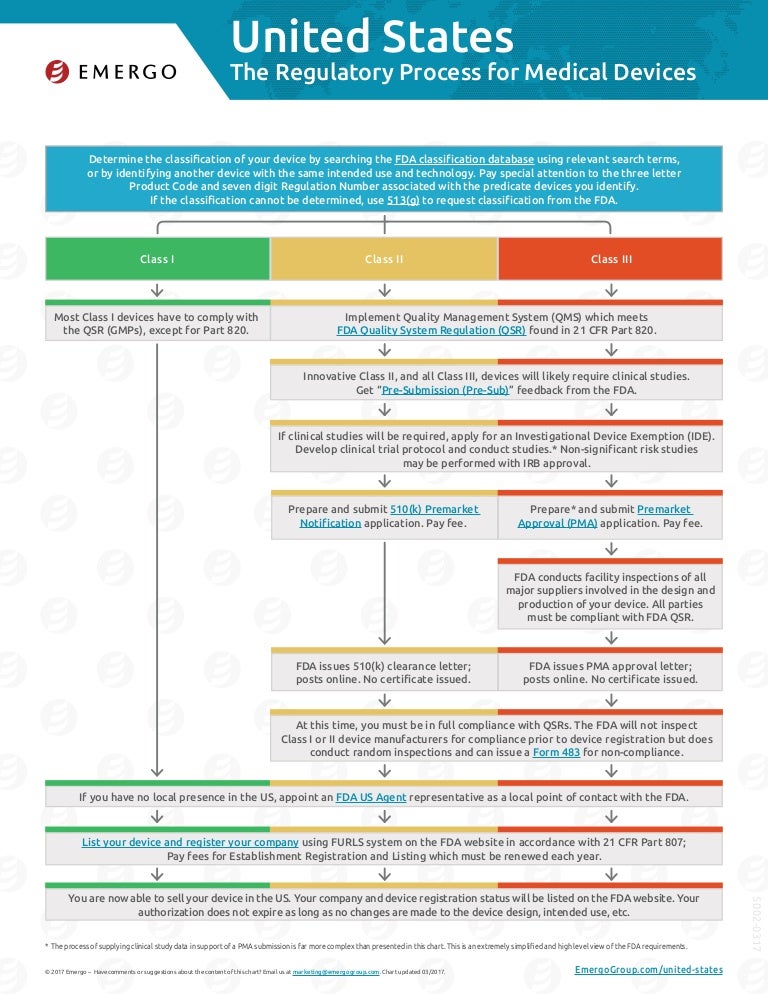
US FDA medical device approval chart Emergo
Fda Approval Calendar Harri Pepita
The Fda’s 2024 Pdufa Calendar Is Far From Complete, But Already It Contains Several Dates Worth Watching, From The First New Drug Class For Schizophrenia In Decades, To A.
See Drugs@Fda For Information About All Of Cder’s Approved Drugs And Biological Products.
We Are In The Process Of Updating Fda.gov Content To Reflect These Changes.
Therapy For Hereditary Angioedema (Hae).
Related Post: