Pharmaceutical Approval Calendar
Pharmaceutical Approval Calendar - Up to date information on the latest fda drug approvals. Pdufa dates (fda approval) for all us publicly listed biotech. Fda novel drug therapy approvals for 2025. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is expected to take. In a phase 3 study, gsk's vaccine—given in two doses six months. Benzinga's fda calendar shows historical fda data,. Get more info on each drug in our drug iq pages including drug history and mechanism of action. The pdufa date refers to the date the food and drug. Includes list of most recent approvals, the conditions approved for, and the approval history. Keep an eye out for the newest drugs and vaccines. Pdufa dates (fda approval) for all us publicly listed biotech. Updated daily, it includes pdufa dates for 2025, fda advisory meetings,. Cder drug and biologic approvals for calendar year 2016; & biologic license application (bla) efficacy. In a phase 3 study, gsk's vaccine—given in two doses six months. Data orgovyx (relugolix) sumitomo pharma switzerland gmbh s 10/18/2024 nda 217806 6 labeling change with clinical. Up to date information on the latest fda drug approvals. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is expected to take. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Dive deeper into stocks you follow with our catalyst calendar and pipeline screener. Fda novel drug therapy approvals for 2025. Cder drug and biologic approvals for calendar year 2015; Get more info on each drug in our drug iq pages including drug history and mechanism of action. Updated daily, the fda calendar gives you insight into fda actions on companies. Includes list of most recent approvals, the conditions approved for, and the approval history. The pdufa date refers to the date the food and drug. Comparing the fda new drug approval lists from 2023 and 2024 reveals a significant difference in the size of the companies that achieved the regulatory triumphs. Get more info on each drug in our drug. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is expected to take. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. Keep an eye out for the newest drugs and vaccines. & biologic license application (bla) efficacy. Obtaining approval from the u.s. Dive deeper into stocks you follow with our catalyst calendar and pipeline screener. Many more will receive approval in 2025. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. In 2021, cder approved 50 new drugs, either as new molecular entities (nmes) under new drug applications (ndas), or as new therapeutic biologics under. Our pdufa calendar includes future pdufa dates for biotech companies, as well as advisory committee meeting dates. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is expected to take. & biologic license. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. In a phase 3 study, gsk's vaccine—given in two doses six months. Comparing the fda new drug approval lists from 2023 and 2024 reveals a significant difference in the size of the companies that achieved the regulatory triumphs. Data orgovyx (relugolix) sumitomo pharma switzerland. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. Obtaining approval from the u.s. Dive deeper into stocks you follow with our catalyst calendar and pipeline screener. Our fda calendar is designed. Benzinga's fda calendar shows historical fda data,. Pdufa dates (fda approval) for all us publicly listed biotech. Several new drugs, vaccines, and otc products received fda approval in 2024. In a phase 3 study, gsk's vaccine—given in two doses six months. Updated daily, the fda calendar gives you insight into fda actions on companies and upcoming actions the fda is. It opens up new growth opportunities. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers companies publicly traded. Our fda calendar is designed to provide you with future. Cder drug and biologic approvals for calendar year 2015; In a phase 3 study, gsk's vaccine—given in two doses six months. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. & biologic license application (bla) efficacy. The pdufa date refers to the date the food and drug. Data orgovyx (relugolix) sumitomo pharma switzerland gmbh s 10/18/2024 nda 217806 6 labeling change with clinical. Comparing the fda new drug approval lists from 2023 and 2024 reveals a significant difference in the size of the companies that achieved the regulatory triumphs. Several new drugs, vaccines, and otc products received fda approval in 2024. Cder drug and biologic approvals for calendar year 2015; Get more info on each drug in our drug iq pages including drug history and mechanism of action. & biologic license application (bla) efficacy. In 2021, cder approved 50 new drugs, either as new molecular entities (nmes) under new drug applications (ndas), or as new therapeutic biologics under biologics license applications. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. Many more will receive approval in 2025. Benzinga's fda calendar shows historical fda data,. Obtaining approval from the u.s. In a phase 3 study, gsk's vaccine—given in two doses six months. Keep an eye out for the newest drugs and vaccines. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers companies publicly traded. Fda novel drug therapy approvals for 2025. Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock.Fda Phase 3 Approval Calendar Lilly Phaidra
Fda Drug Approval Calendar
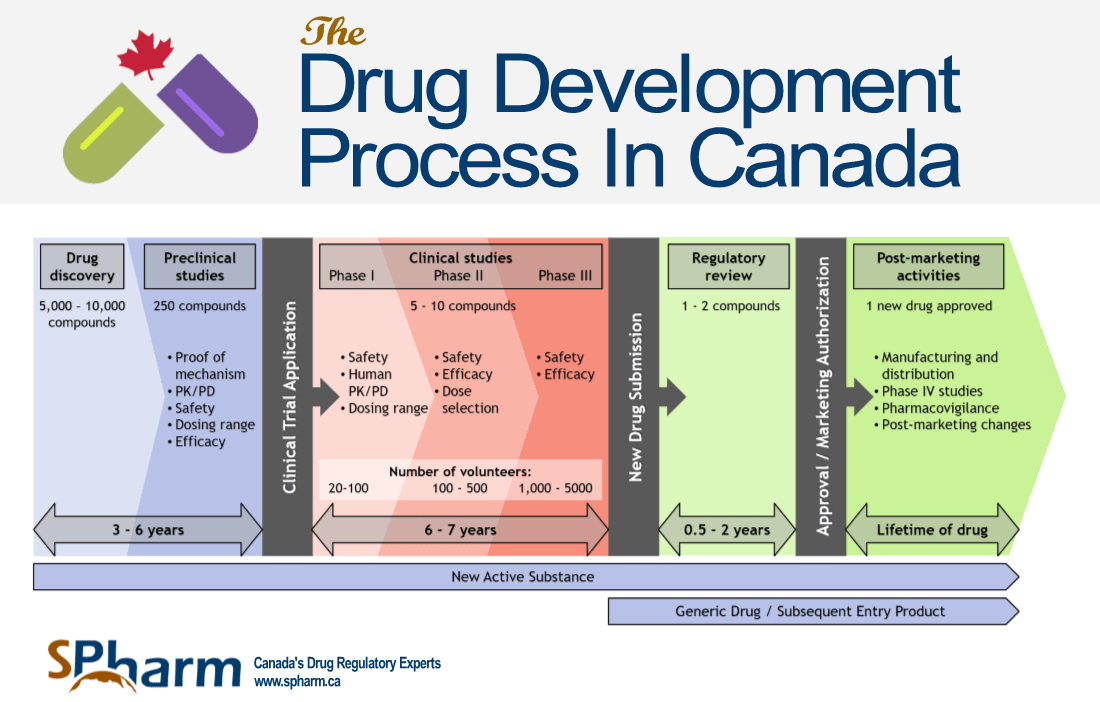
FDA Drug Approval Process Timeline
Fda Drug Approval Calendar
FDA Calendar FDA Tracker
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Fda Drug Approval Calendar
Fda Approval Calendar Harri Pepita
Fda Drug Approval Calendar
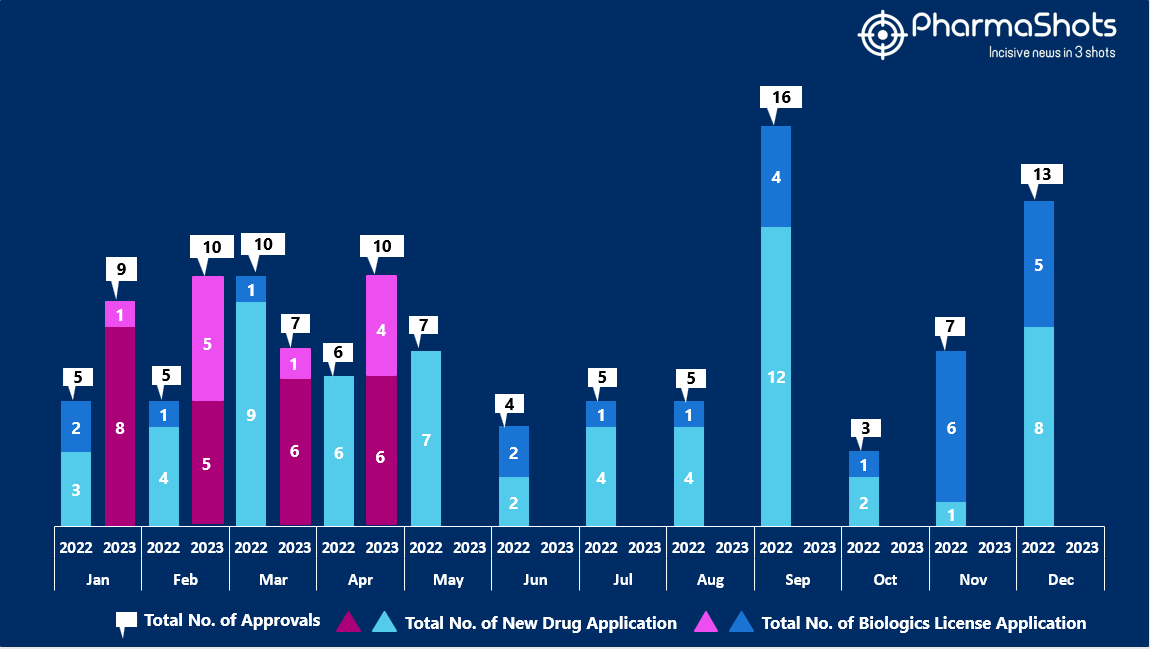
Insights+ The US FDA New Drug Approvals in April 2023
Up To Date Information On The Latest Fda Drug Approvals.
The Pdufa Date Refers To The Date The Food And Drug.
Updated Daily, It Offers Investors Insights Into Stock.
With Our Free Fda Calendar, Track Upcoming Pdufa Dates, Fda Approvals, Biotech Catalysts, Clinical Trials, And Regulatory Events.
Related Post: