Pharmaceutical Fda Approval Calendar
Pharmaceutical Fda Approval Calendar - Sign up or log in to access our enhanced fda calendar! (2) application has been tentatively approved or approved under pepfar. It opens up new growth opportunities. Cder drug and biologic approvals for calendar. Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock. Fda novel drug therapy approvals for 2025. Get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews As europe’s top biotech website for over a decade, labiotech.eu offers trusted insights and updates for the global life sciences community. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Obtaining approval from the u.s. Cder drug and biologic approvals for calendar year 2019; The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. Sign up or log in to access our enhanced fda calendar! Obtaining approval from the u.s. In looking at the product mix of new drug approvals, of the 50 new drug approvals by fda’s cder in 2024, 34 or 68%, were small molecules (31 drugs and three. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for therapeutic purposes) drug approval. Fda approval of sublocade label changes delayed february 12, 2025 02:32 et. Fda novel drug therapy approvals for 2025. Includes list of most recent approvals, the conditions approved for, and the approval history. Approval information by product type drugs. Subscribe to our newsletter to get. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for therapeutic purposes) drug approval. Cder drug and biologic approvals for calendar year 2020; On february 11, 2025, the food and drug administration approved brentuximab vedotin (adcetris, seagen inc., a subsidiary of pfizer) in combination with lenalidomide and a. The. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for therapeutic purposes) drug approval. It opens up new growth opportunities. Get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews (2) application has been tentatively approved or approved under pepfar. Fda approval of sublocade label changes delayed. Indivior plc (indv, indv.l), a specialty pharmaceutical company, announced. Cder drug and biologic approvals for calendar year 2020; Approval information by product type drugs. As europe’s top biotech website for over a decade, labiotech.eu offers trusted insights and updates for the global life sciences community. In looking at the product mix of new drug approvals, of the 50 new drug. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. On february 11, 2025, the food and drug administration approved brentuximab vedotin (adcetris, seagen inc., a subsidiary of pfizer) in combination with lenalidomide and a. Food and drug administration (fda) for. Approval information by product type drugs. Cder drug and biologic approvals for calendar year 2020; Cder drug and biologic approvals for calendar year 2019; Includes list of most recent approvals, the conditions approved for, and the approval history. This calendar tracks upcoming pdufa drug approval dates and fda advisory committee meetings. Up to date information on the latest fda drug approvals. Get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. The prescription drug user fee act. Cder drug and biologic approvals for calendar. Fda approval of sublocade label changes delayed february 12, 2025 02:32 et. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. The prescription drug user fee act (pdufa) date refers to the deadline set by the. Get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock. The us food and drug administration (fda) has approved springworks therapeutics’ gomekli (mirdametinib) to treat neurofibromatosis type 1 (nf1), a type of rare. Our. Fda novel drug therapy approvals for 2025. Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock. Cder drug and biologic approvals for calendar year 2019; Cder drug and biologic approvals for calendar. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for therapeutic purposes) drug approval. Get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews This calendar tracks upcoming pdufa drug approval dates and fda advisory committee meetings. On february 11, 2025, the food and drug administration approved brentuximab vedotin (adcetris, seagen inc., a subsidiary of pfizer) in combination with lenalidomide and a. Indivior plc (indv, indv.l),. Obtaining approval from the u.s. Cder drug and biologic approvals for calendar year 2020; Fda approval of sublocade label changes delayed february 12, 2025 02:32 et. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. The us food and drug administration (fda) has approved springworks therapeutics’ gomekli (mirdametinib) to treat neurofibromatosis type 1 (nf1), a type of rare. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. It opens up new growth opportunities. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Cder drug and biologic approvals for calendar year 2019; Subscribe to our newsletter to get. Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock. Up to date information on the latest fda drug approvals. Indivior plc (indv, indv.l), a specialty pharmaceutical company, announced. On february 11, 2025, the food and drug administration approved brentuximab vedotin (adcetris, seagen inc., a subsidiary of pfizer) in combination with lenalidomide and a. Sign up or log in to access our enhanced fda calendar!Cancer Immunotherapy in Diffuse Large BCell Lymphoma. Abstract
Customized Table and wall Calender Designing and Printing
FDA 510k Submission Process & Guidance 🥇I3CGlobal
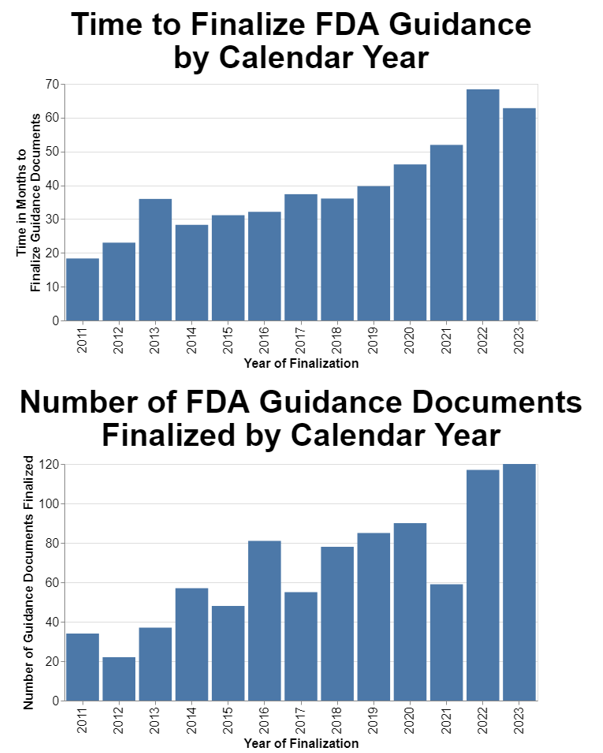
Unpacking Averages Analyzing FDA’s Performance in Finalizing Guidance
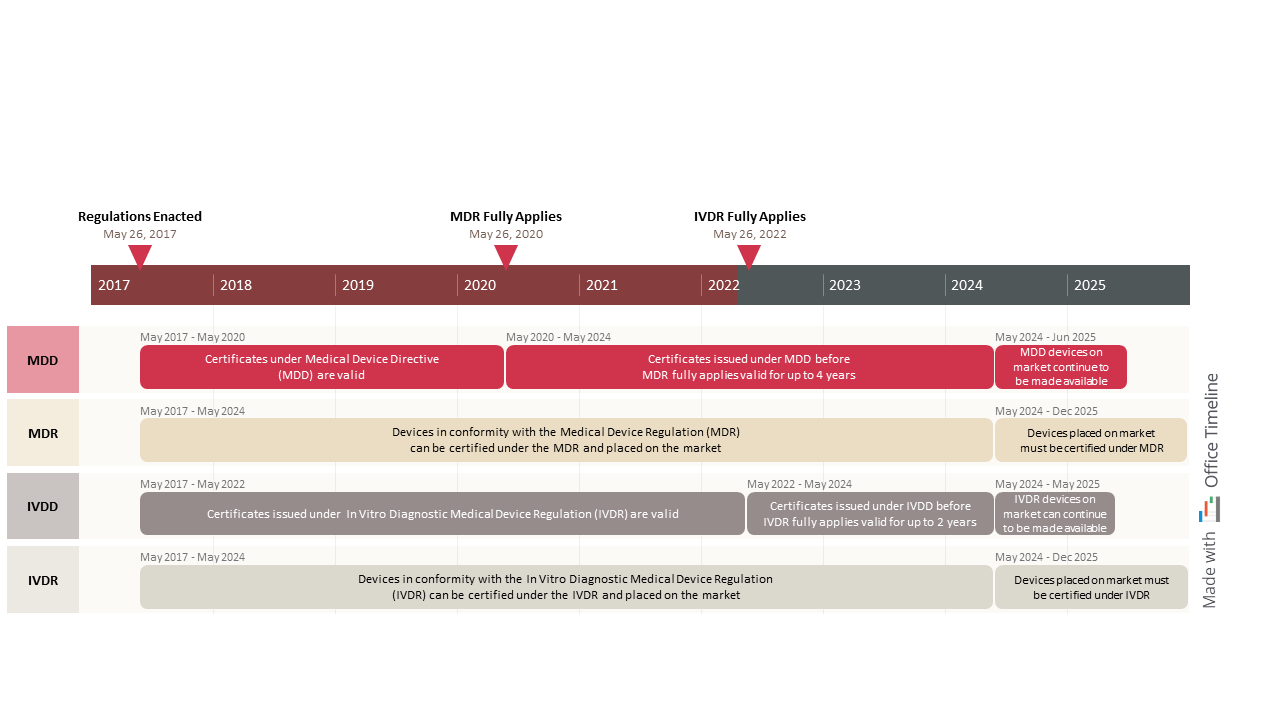
Updated timeline for the use of eAFs for variations in human drugs EXTEDO
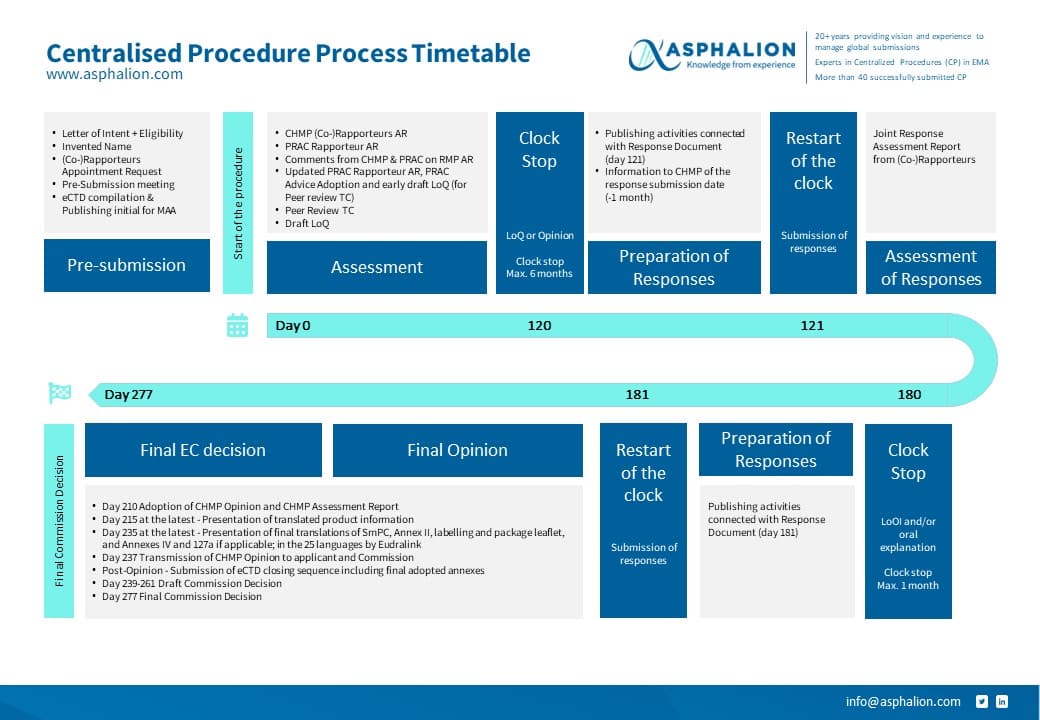
New CENTRALISED PROCEDURE PROCESS FLOW CHART
FDA カレンダーフォトコンテスト 2022 入選作品のご紹介 航空券予約・購入はフジドリームエアラインズ(FDA)
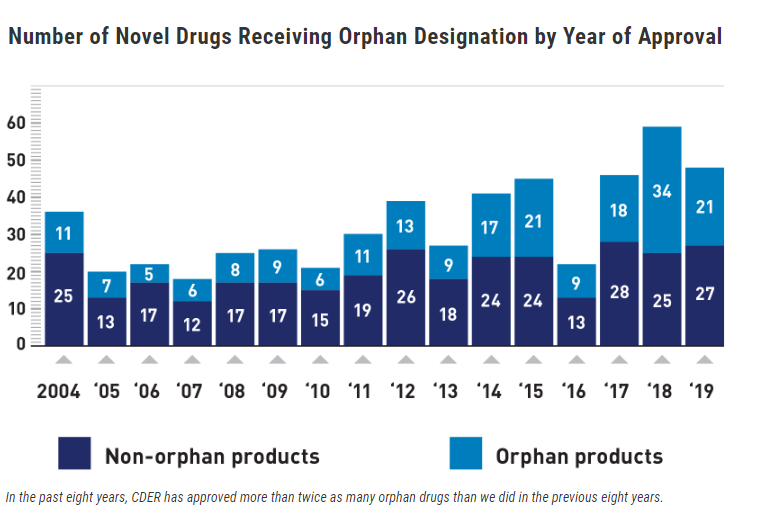
FDA 2019 Continues Uptick in Orphan Drug Approvals RAPS
Top examples of timelines, Gantt charts, and roadmaps for the Pharma
The Most Important New Drug Of 2014
(2) Application Has Been Tentatively Approved Or Approved Under Pepfar.
Fda Novel Drug Therapy Approvals For 2025.
Includes List Of Most Recent Approvals, The Conditions Approved For, And The Approval History.
In Looking At The Product Mix Of New Drug Approvals, Of The 50 New Drug Approvals By Fda’s Cder In 2024, 34 Or 68%, Were Small Molecules (31 Drugs And Three.
Related Post: