Pharmaceutical Approcall Calendar
Pharmaceutical Approcall Calendar - Fda novel drug therapy approvals for 2024. Pain is a common medical problem and relief of pain is an important therapeutic. Below is the list of important regulatory dates for all. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers companies publicly traded. It opens up new growth opportunities. Some of the notable drug approvals that occurred in january 2025. Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock. Obtaining approval from the u.s. Almost half of all novel medications approved by the u.s. These include clinical trials, commercial, and fda or other regulatory events from the. What is an fda calendar? Some of the notable drug approvals that occurred in january 2025. Cy 2024 cder drug and biologic calendar year approvals. Updated daily, it offers investors insights into stock. Updated daily, it includes pdufa dates for 2025, fda advisory meetings,. Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock. The table below is a running list of cder’s novel drugs approvals for 2024. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Pdufa dates (fda approval) for all us publicly listed biotech. Up to date information on the latest fda drug approvals. Pain is a common medical problem and relief of pain is an important therapeutic. Some of the notable drug approvals that occurred in january 2025. Below is the list of important regulatory dates for all. Cy 2024 cder drug and biologic calendar year approvals. It opens up new growth opportunities. Get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews Some of the notable drug approvals that occurred in january 2025. Despite promising phase 3 results, safety concerns, namely liver injury, have impacted agios stock and could affect pyrukund's approval and market adoption. A list of. The catalyst calendar is a chronological calendar of biotech events that could move the stock price. Includes list of most recent approvals, the conditions approved for, and the approval history. It opens up new growth opportunities. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. These include clinical trials, commercial, and. Therapy for hereditary angioedema (hae). Food and drug administration (fda) are orphan drugs. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Pain is a common medical problem and relief of pain is an important therapeutic. Therapy for hereditary angioedema (hae). Up to date information on the latest fda drug approvals. Get the latest information on. The table below is a running list of cder’s novel drugs approvals for 2024. Despite promising phase 3 results, safety concerns, namely liver injury, have impacted agios stock and could affect pyrukund's approval and market adoption. Nda 213976 eohilia budesonide oral suspension takeda pharmaceuticals usa inc p,o 2/9/2024 y. Cder drug and biologic approvals for calendar year 2015; Some of. It opens up new growth opportunities. What is an fda calendar? Updated daily, it offers investors insights into stock. Pain is a common medical problem and relief of pain is an important therapeutic. To treat unresectable or metastatic,. Nda 213976 eohilia budesonide oral suspension takeda pharmaceuticals usa inc p,o 2/9/2024 y. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers companies publicly traded. Journavx is the first drug to be approved in this new class of pain management medicines. Includes list of most recent. Food and drug administration (fda) for a key medication can be a huge catalyst for a healthcare stock. Despite promising phase 3 results, safety concerns, namely liver injury, have impacted agios stock and could affect pyrukund's approval and market adoption. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Therapy. Fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. It opens up new growth opportunities. The catalyst calendar is a chronological calendar of biotech. Almost half of all novel medications approved by the u.s. The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). Food and drug administration authorized the marketing of 20 zyn nicotine pouch products through the premarket tobacco product application (pmta) pathway. Updated daily, it includes pdufa dates for 2025, fda advisory meetings,. Journavx is the first drug to be approved in this new class of pain management medicines. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application (nda) or. Fda novel drug therapy approvals for 2024. With our free fda calendar, track upcoming pdufa dates, fda approvals, biotech catalysts, clinical trials, and regulatory events. Get the latest information on clinical trials, fda drug approvals, fda calendar, fda events and more on rttnews Pain is a common medical problem and relief of pain is an important therapeutic. Updated daily, it offers investors insights into stock. Our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Access our free pdufa calendar to track upcoming pdufa dates, fda approval dates, and biotech catalysts. What is an fda calendar? To treat unresectable or metastatic,. Includes list of most recent approvals, the conditions approved for, and the approval history.Fda Approval Calendar Harri Pepita
FDA Calendar FDA Tracker
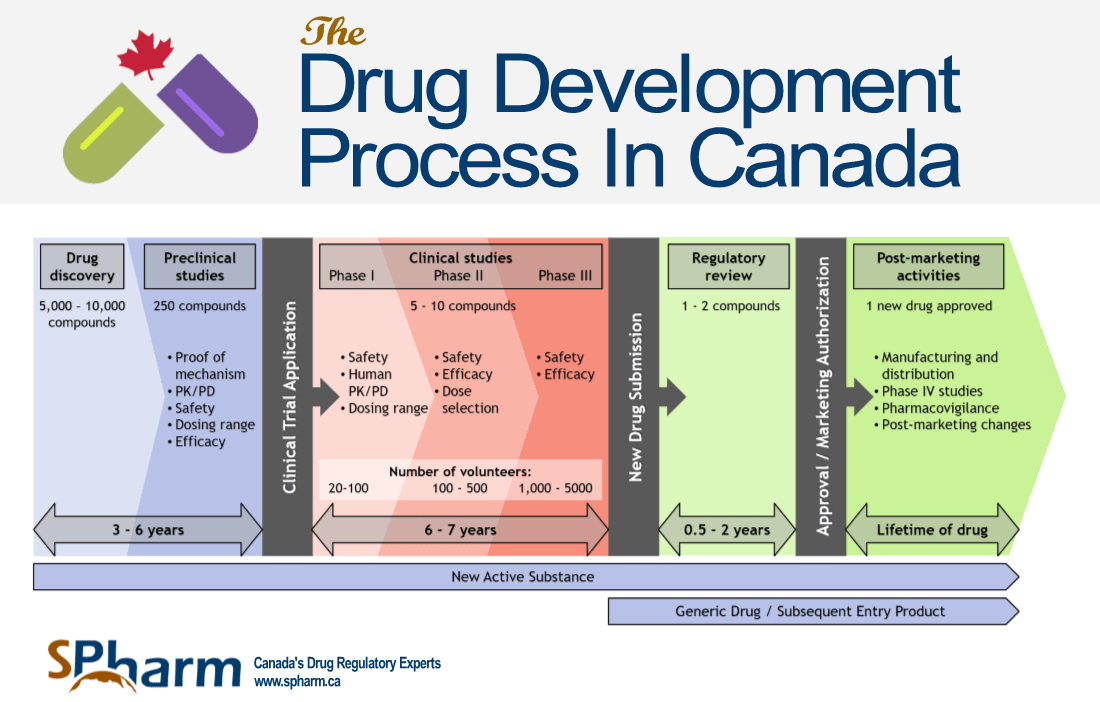
FDA Drug Approval Process Timeline
Fda Drug Approval Calendar
Fda Drug Approval Calendar
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Insights+ The US FDA New Drug Approvals in June 2022
Fda Drug Approval Calendar Printable Word Searches
Fda Drug Approval Calendar
Fda Phase 3 Approval Calendar Lilly Phaidra
Pdufa Dates (Fda Approval) For All Us Publicly Listed Biotech.
It Opens Up New Growth Opportunities.
Below Is The List Of Important Regulatory Dates For All.
Therapy For Hereditary Angioedema (Hae).
Related Post: